The Trial Master File, or TMF, is the compilation of essential documents that permits the evaluation of the conduct of a clinical trial and the quality of the data produced. It is structured to allow an independent reconstruction of the trial, demonstrating that the study was conducted in compliance with Good Clinical practice (GCP) standards and applicable regulatory requirements. The TMF serves as a primary resource during regulatory inspections.
What Is a Trial Master File
If a regulatory inspector were to request documentation justifying a critical decision made during a trial, the TMF is the official repository for that information. The TMF is a dynamic collection of documents that evolves throughout the study lifecycle, from the initial protocol draft to the final clinical study report.
The objective of the TMF is to enable an independent auditor or regulatory authority to reconstruct the history of the trial. This collection demonstrates data integrity, adherence to the protocol, and protection of trial participants' rights and safety. The TMF is composed of core regulatory documents in clinical trials.
The Core Purpose of the TMF
The primary purpose of the TMF is to demonstrate regulatory compliance. It is a mandated requirement established by authorities such as the International Council for Harmonisation (ICH) within its Good Clinical Practice guidelines.
The fundamental role of the TMF is to provide a clear, auditable trail that validates how the trial was conducted and the quality of the data it generated. A complete and accurate TMF allows for the independent evaluation of a study’s scientific and ethical integrity.
A well-maintained TMF fulfills several key functions:
- Demonstrates Compliance: It serves as primary evidence that the sponsor and investigators met their regulatory obligations.
- Facilitates Audits and Inspections: It provides authorities like the FDA or EMA a structured means to review and verify all aspects of the trial.
- Ensures Data Credibility: It supports the validity of trial data, which is fundamental to the regulatory approval process for a new therapeutic.
- Supports Trial Management: It functions as a central repository for the study team, facilitating the management of trial activities.
The Shift to Electronic Systems
Historically, the TMF was a collection of physical paper records, often occupying entire rooms. This format presented significant logistical challenges related to document updates, version control, and providing access to a global study team.
The industry has largely transitioned to the electronic Trial Master File (eTMF) to address these operational inefficiencies. This transition reflects the need for a more efficient, secure, and accessible method for managing the extensive documentation generated by modern clinical trials.
The global eTMF systems market was valued at USD 1.21 billion in 2024 and is projected to reach USD 2.49 billion by 2030, indicating the critical role these platforms now play. Digitalization helps embed compliance and inspection readiness into trial operations from the outset.
Navigating the TMF Regulatory Landscape
The establishment and maintenance of a Trial Master File are governed by a framework of global and regional regulations. These regulations are designed to protect patient safety and ensure the integrity of clinical trial data. The TMF provides the evidence that a trial was conducted ethically and scientifically.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) sets the global standard. Its ICH E6 Good Clinical Practice (GCP) guideline is the foundational document for TMF requirements.
ICH E6 stipulates that sponsors and investigators must maintain a set of essential documents which, individually and collectively, permit evaluation of the conduct of a trial and the quality of the data produced. The TMF must be established at the beginning of the trial and updated throughout its duration, which is essential for effective trial management.
The Role of ICH E6 Good Clinical Practice
ICH E6 delineates documentation responsibilities between the sponsor and the investigator. While their records have some overlap, each party maintains documents pertinent to their respective roles.
- Sponsor TMF: This is the comprehensive collection containing all trial-related documents from all participating sites. It provides a complete overview of the entire study, from the protocol to the final clinical study report.
- Investigator TMF (or Investigator Site File – ISF): This is a site-specific file containing documents relevant to a particular investigator and their team, enabling them to manage their site-level activities and demonstrate compliance.
The guideline emphasizes that the TMF must be complete, accurate, and contemporaneous. The term "contemporaneous" signifies that documents should be filed as they are generated, creating a real-time record of trial activities rather than a historical archive assembled at the study's conclusion.
Regional Regulatory Requirements
While ICH provides the global framework, health authorities like the FDA and EMA have specific requirements, particularly concerning electronic systems.
Regulatory bodies like the FDA and EMA require not just a complete TMF, but also verifiable proof of its integrity. This necessitates demonstrable control over electronic records, signatures, and a clear audit trail showing who performed an action, when it was done, and why.
For trials involving the United States, 21 CFR Part 11 is a key regulation. This FDA regulation defines the criteria under which electronic records and signatures are considered trustworthy, reliable, and legally equivalent to paper records. To comply, an eTMF system must have robust access controls, detailed audit trails, and proper procedures for electronic signatures.
In Europe, the European Medicines Agency (EMA) has issued detailed guidance on TMF management. The EMA emphasizes a risk-based approach, encouraging teams to focus quality control efforts on documents and processes most critical to patient safety and data integrity. These regulations provide the direct link between daily filing activities and the fundamental goal of conducting a credible clinical trial.
How to Structure Your TMF for Clarity and Compliance
An organized Trial Master File is an inspectable TMF. Without a logical structure, a TMF can become a disorganized collection of documents, making it difficult for an auditor to reconstruct the trial. Adopting a standardized filing system that is understood by all stakeholders—from internal teams to inspectors—is key to avoiding this issue.
This is analogous to a library's cataloging system; a universal structure allows any user to locate information efficiently. For clinical trials, the industry standard is the TMF Reference Model.
Introducing the TMF Reference Model
The TMF Reference Model provides a standardized taxonomy for organizing TMF documents. It was developed by the industry to promote order and consistency in trial documentation. This means a document concerning a site initiation visit, for example, is filed in the same logical location regardless of the sponsor, CRO, or eTMF system in use.
The model organizes trial documents into logical categories called zones. Each zone represents a distinct functional area of the study, which makes locating and filing documents more intuitive and efficient. By mapping every essential document to a specific zone, section, and artifact, the model creates a clear, predictable structure for navigating the TMF.
The diagram below illustrates the hierarchy of TMF regulations, from global guidelines down to the specific roles that determine document ownership and filing responsibilities.
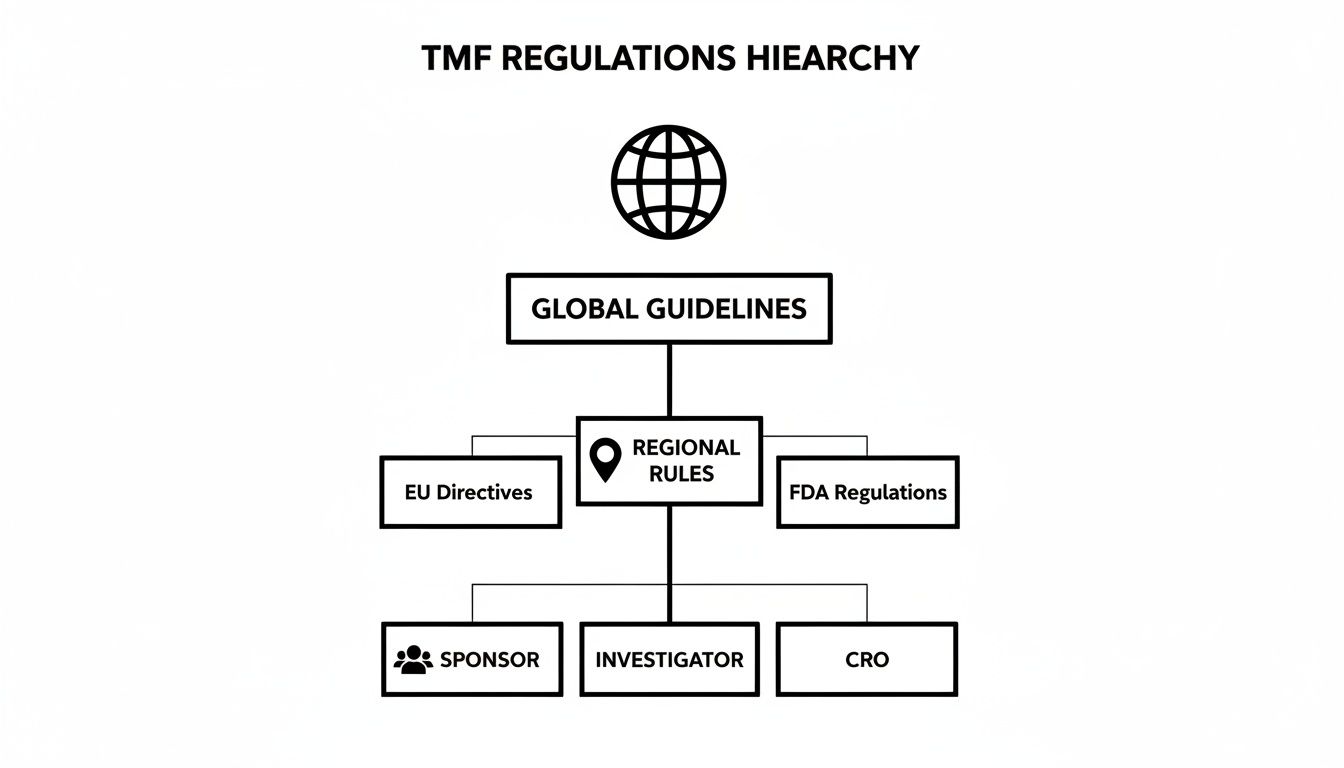
This hierarchy demonstrates that TMF structure is not arbitrary. It reflects a well-defined regulatory framework designed to ensure consistency and compliance across all trials.
Key Zones Within the Reference Model
The TMF Reference Model is organized into several high-level zones, each containing documents, or "artifacts," relevant to that aspect of the trial. Understanding these zones is the first step toward building a TMF that is both compliant and inspection-ready.
The table below provides an overview of the TMF Reference Model, highlighting its core zones and examples of documents found within each.
| Zone Number | Zone Name | Example Documents |
|---|---|---|
| 01 | Trial Management | TMF Plan, Project Management Plans, Key Decision Logs, Meeting Minutes |
| 02 | Central Trial Documents | Protocol and Amendments, Investigator's Brochure (IB), Investigational Product records |
| 03 | Regulatory | Regulatory Authority Submissions, Agency Correspondence, Annual Safety Reports |
| 04 | Site Management | Site Feasibility Questionnaires, Monitoring Visit Reports, Site Communication Logs |
| 05 | IRB/IEC and Other Approvals | IRB/IEC Submissions and Approvals, Informed Consent Form (ICF) versions, Patient-facing materials |
| 06 | Non-Clinical | Bioanalytical Reports, Toxicology Study Reports |
| 07 | Investigational Product | Drug Accountability Records, Shipping and Temperature Logs, Certificates of Analysis |
| 08 | Clinical Trial | Subject Screening and Enrollment Logs, Signed ICFs, Source Document Worksheets |
| 09 | Data Management | Data Management Plan, eCRF Completion Guidelines, Database Lock Documentation |
| 10 | Statistics | Statistical Analysis Plan (SAP), Programming Specifications, Mock Tables, Listings, and Figures |
| 11 | Trial Committees | Data Safety Monitoring Board (DSMB) Charter, Steering Committee Meeting Minutes |
Understanding this structure transforms the TMF from a simple repository into a dynamic tool that allows auditors to follow the trial's narrative.
A standardized structure does more than simplify filing; it fosters a state of continuous readiness. When all stakeholders adhere to the same organizational framework, internal quality checks, CRO oversight, and regulatory inspections become significantly more streamlined.
This common language for TMF organization facilitates modern trial collaboration. The TMF Reference Model, developed to address fragmented filing systems, has become a widely adopted standard. It enables seamless integration between sponsors and CROs and ensures an inspector-friendly consistency that is vital during audits. This is particularly beneficial for smaller biotech companies with limited resources, as it helps avoid inefficiencies that can arise when multiple teams contribute to the TMF without a unified framework. Additional guidance is available for achieving TMF inspection readiness.
Moving from Paper to Electronic TMF Systems
The transition from paper-based systems to digital platforms represents a fundamental shift in how clinical trial documentation is managed, accessed, and protected. For years, the paper Trial Master File was the standard, but it has become a source of significant operational challenges in today's complex, global trials.
A paper TMF is constrained by physical logistics. Locating a specific document can be time-consuming. Version control relies on manual logs, and providing simultaneous access to a global team of monitors, sites, and CROs is impractical. This legacy approach often leads to information bottlenecks and delays, making real-time oversight difficult to achieve.
Electronic TMF (eTMF) systems were developed to resolve these issues. By consolidating all trial documents into a single, secure digital environment, an eTMF provides the necessary infrastructure for modern, compliant trial management.
Operational Advantages of eTMF Platforms
The most immediate benefit of an eTMF is providing authorized personnel with real-time access to the complete Trial Master File, regardless of their location. This visibility allows teams to be proactive, monitoring the health and completeness of their TMF on an ongoing basis rather than during periodic reviews.
Modern eTMF systems also introduce automation. They can track expected documents, send reminders for overdue items, and manage review and approval workflows. This reduces the administrative burden on clinical teams and helps enforce the GCP principle of contemporaneous filing.
From a regulatory standpoint, a key feature is the unchangeable audit trail. Every action—such as an upload, signature, or view—is automatically recorded with a user, date, and timestamp. This provides inspectors with a transparent history of TMF activities, which is difficult to demonstrate with a paper-based system. You can explore the capabilities of a dedicated electronic trial master file software in our detailed guide.
To illustrate the differences, the following is a side-by-side comparison of paper and electronic systems.
Comparing Paper TMF and Electronic TMF (eTMF) Operations
| Attribute | Paper TMF | Electronic TMF (eTMF) |
|---|---|---|
| Accessibility | Limited to one physical location. Simultaneous access is impossible. | Global, simultaneous access for all authorized users. |
| Document Retrieval | Manual, slow, and labor-intensive. | Instantaneous, with powerful search and filtering capabilities. |
| Version Control | Manual, prone to human error, and difficult to track. | Automated, ensuring everyone is working from the current version. |
| Collaboration | Difficult. Requires shipping documents or scheduled onsite visits. | Seamless. Real-time review, commenting, and approvals. |
| Audit Trail | Incomplete and relies on manual logs. Difficult to verify. | Automatic, comprehensive, and uneditable. Fully transparent. |
| Inspection Readiness | Requires extensive manual preparation and physical logistics. | Always "inspection-ready" with instant access for auditors. |
| Security | Vulnerable to physical damage (fire, flood) and unauthorized access. | Robust security with role-based permissions and data encryption. |
As the table shows, an eTMF does not just digitize legacy processes—it introduces a more efficient, secure, and compliant method of operation.
Addressing the Challenges of Digital Transition
While the benefits are clear, transitioning from paper to an eTMF requires careful planning. Migrating historical documents from past or ongoing studies into a validated digital system is a significant project. If not managed properly, it can lead to operational risks.
A poorly executed migration can result in corrupted files, lost data, or an incomplete digital record. Therefore, a validated migration plan is essential. This plan must detail the entire process: scanning, indexing, and performing quality checks to verify that the digital copies are accurate representations of the originals.
Migrating legacy Trial Master Files (TMFs) to centralized eTMF systems is a critical yet challenging process for pharma companies and CROs aiming for efficiency and compliance, but data reveals significant pitfalls that demand meticulous planning.
Data from a 2017 study highlight the complexities of this process. It found that 31% of migration projects failed completely, and another 54% of completed projects did not meet their stated objectives. These statistics underscore the risks, from data loss to workflow disruption. A methodical, risk-based approach to TMF transition is therefore a necessity.
Keeping Your TMF Ready for Inspection, All the Time

Inspection readiness is not a state achieved immediately prior to an audit. It is the outcome of a continuous commitment to quality that is integrated into trial operations from the beginning.
A healthy Trial Master File is not assembled in response to an upcoming inspection. It is a living record that is methodically built and maintained throughout the study, ensuring it accurately reflects the trial's progress in real time.
This proactive approach mitigates the last-minute stress and remediation efforts that many teams face. When quality checks and clear responsibilities are part of standard procedure, a state of constant preparedness is maintained. The goal is to make readiness a standard operating procedure, not an emergency response.
It All Starts with a Solid TMF Plan
The cornerstone of a healthy TMF is a comprehensive TMF Plan. This document serves as the strategic blueprint for the entire documentation process and is developed at study startup. It should define the who, what, when, where, and how for every document intended for the TMF.
An effective TMF Plan will clearly delineate:
- Ownership and Responsibilities: It explicitly assigns individuals or teams responsible for creating, reviewing, approving, and filing each document type, establishing clear accountability.
- Filing Timelines: The plan sets realistic deadlines for filing documents, reinforcing the regulatory expectation for contemporaneous documentation.
- Quality Control (QC) Procedures: It defines the frequency and scope of quality checks (e.g., sampling strategies) and the process for remediating any identified issues.
- System and Structure: The plan should identify the eTMF system being used and confirm the filing structure, which is typically based on the industry-standard DIA Trial Master File Reference Model. You can learn more about how to apply the DIA TMF Reference Model in our guide.
The TMF Plan is a living document that must be updated to reflect any changes in the study, such as a protocol amendment or the addition of a new vendor.
Using ALCOA+ to Guarantee Document Integrity
The ALCOA+ principles serve as a framework for ensuring that individual documents meet regulatory standards for data integrity. Originally developed for Good Manufacturing Practice (GMP), ALCOA+ is now a standard for data integrity in GCP and TMF management.
The ALCOA+ framework can be viewed as the lens through which a regulator examines documentation. Each principle is a test of data integrity, and together, they confirm that the TMF provides a trustworthy and complete account of the trial.
Adherence to these principles is non-negotiable for maintaining a high-quality, defensible Trial Master File.
Breaking Down the ALCOA+ Framework
Each letter in the ALCOA+ acronym represents a critical quality attribute:
- Attributable: Can the individual who created or modified a record and the time of the action be identified? This is accomplished through signatures and dates or tracked automatically by an eTMF's audit trail.
- Legible: Is the information readable and permanent? This applies to both handwritten source documents and electronic records.
- Contemporaneous: Was the information recorded at the time the activity occurred? Backdating is a serious compliance issue that undermines a document's credibility.
- Original: Is the record the first place the data was recorded, or is it a certified true copy?
- Accurate: Does the data reflect what actually happened? It must be correct, truthful, and precise.
The “+” was added to include several other essential qualities:
- Complete: Does the record include all relevant data, including documentation of repeated analyses or failed attempts?
- Consistent: Is the sequence of events chronological and logical across all related documents?
- Enduring: Will the record remain intact and accessible for the entire required retention period?
- Available: Can the record be accessed for review, audit, or inspection upon request?
By combining regular QC reviews (as defined in the TMF Plan) with a document-level assessment against ALCOA+ principles, TMF management becomes an active quality assurance function. This continuous oversight is key to ensuring a constant state of inspection readiness.
How Quality Authoring Builds a Stronger TMF Foundation
The integrity of a Trial Master File begins not at the time of filing, but at the time of authoring. The quality of the TMF is fundamentally dependent on the quality of its core documents, such as the protocol, the Investigator's Brochure (IB), and the Clinical Study Report (CSR).
Any error, inconsistency, or ambiguity in these foundational documents can create downstream compliance risks and require significant remediation efforts during TMF quality control (QC) reviews.
This perspective shifts TMF management from a reactive filing task to a proactive, quality-focused process. When study teams prioritize quality from the initial draft, they ensure that the documents constituting the trial record are accurate, complete, and compliant from their inception. This approach builds the TMF on a solid foundation.
Embedding Compliance While You Write
Modern clinical documentation platforms can integrate compliance-focused workflows into the authoring process. Instead of starting with a blank template, teams can work within a structured environment where regulatory intelligence is embedded.
For instance, when drafting a protocol, a structured authoring tool can guide the user through each required section based on ICH guidelines, reducing the likelihood of omitting critical information. For an IB, the system can enforce version control and track safety updates, ensuring the document remains current. This makes compliance the path of least resistance.
The quality of your TMF is a direct reflection of the quality of the documents it contains. Focusing on excellence during authoring is the most direct path to continuous inspection readiness, as it prevents errors from entering the file in the first place.
From First Draft to Audit-Ready
This "upstream" focus on quality produces documents that are substantially closer to being audit-ready from the start. When a protocol or CSR is created using a controlled, template-driven process, the final output is standardized and predictable.
This consistency simplifies the work of TMF managers, QA teams, and regulatory inspectors who must review the documentation.
This approach helps to eliminate common issues identified during TMF reviews, such as:
- Inconsistent Terminology: Ensuring a term like "adverse event" is used consistently across all related documents.
- Version Mismatches: Preventing an outdated protocol version from being referenced in a new informed consent form.
- Missing Information: Ensuring all regulatory-required sections are included and completed before a document is finalized.
By integrating quality directly into the authoring process, you create a more resilient and defensible TMF. The documentation process is transformed from a potential liability into a strategic asset for achieving regulatory success.
Your TMF Questions, Answered
This section addresses some of the most common questions regarding the management of a Trial Master File.
TMF vs. eTMF: What’s the Real Difference?
The Trial Master File (TMF) is the complete collection of essential documents for a clinical trial, which demonstrates compliance with the protocol, patient safety measures, and data integrity. The term itself refers to the collection of documents, regardless of the storage medium.
An electronic Trial Master File (eTMF) is the digital manifestation of the TMF. It is a specialized software system designed to manage these documents electronically. The primary advantage of an eTMF extends beyond eliminating paper; it provides real-time global access, enables automated workflows, and maintains a complete, unchangeable audit trail—features that are difficult to achieve with a paper-based system.
Who's Ultimately on the Hook for the TMF?
Ultimately, the sponsor holds the final responsibility for the TMF. According to ICH E6(R3) guidelines, this responsibility is non-delegable.
Even when trial activities are outsourced to a Contract Research Organization (CRO), accountability remains with the sponsor. While the CRO will manage day-to-day TMF activities and contribute documents, the sponsor must maintain active oversight. This includes conducting regular reviews to ensure the TMF is complete, accurate, and inspection-ready at all times.
Can Some Documents Live Outside the TMF?
Yes, but this must be managed deliberately and documented. While the objective is for the eTMF to be the single source of truth, it is common for certain documents to be stored in other validated systems. For example, monitoring visit reports may reside in a Clinical Trial Management System (CTMS), while patient data is captured in an electronic data capture (EDC) system.
The key to this approach is the TMF Plan. This document serves as the master index for all trial documentation. It must clearly specify the location of every essential document, whether it is in the eTMF or another system.
If an inspector requests a document, the TMF Plan should provide a clear pointer to its location. This ensures that the entire trial can be reconstructed, regardless of where individual documents are stored.
What Does "Contemporaneous" Actually Mean for a TMF?
In the context of Good Clinical Practice (GCP), "contemporaneous" is a fundamental principle. It means that documents should be filed in the TMF as soon as the event they document occurs. This "file as you go" approach is in contrast to batch-filing documents at milestones.
This practice is critical for several reasons:
- Ensures Accuracy: Information is filed while it is fresh, reducing the risk of errors or omissions.
- Provides Real-Time Oversight: An up-to-date TMF allows for real-time monitoring of trial progress and status.
- Maintains Inspection Readiness: If the TMF is always current, the team is always prepared for an unannounced inspection. The TMF serves as a living reflection of the trial.
Failure to maintain a contemporaneous TMF is a common and serious finding cited by regulators during inspections.