In a clinical trial, ambiguity regarding roles and responsibilities can lead to significant delays and compliance risks. A structured framework like the RACI model is essential for managing clinical trial documentation. It ensures every task, from the initial drafting of a protocol to the final approval of a clinical study report (CSR), has a clearly defined owner. This clarity helps prevent operational delays and maintains accountability across sponsor, CRO, and vendor teams.
The Operational Necessity of Clear Roles in Clinical Documentation
In the highly regulated environment of clinical trials, unclear role definitions for critical documents such as the protocol, Investigator's Brochure (IB), or CSR can create significant operational and regulatory challenges. A lack of clear ownership is a primary source of inefficiency and a common cause of regulatory findings.
Without defined roles, review cycles can become prolonged as feedback loops remain open, and no single individual has the authority to finalize a document. This can also compromise document quality, as multiple contributors operating without a designated lead may produce inconsistent content. Most importantly, a lack of clear accountability undermines inspection readiness.
The RACI Model for Structured Accountability
The RACI model provides a framework to address these challenges. RACI stands for Responsible, Accountable, Consulted, and Informed, offering a straightforward method for delineating roles in complex projects with numerous stakeholders. It is a practical tool for establishing a structured, auditable process for document lifecycle management.
The components are defined as follows:
- Responsible: The individual(s) who perform the work, such as a medical writer drafting a section of the protocol.
- Accountable: The single individual with ultimate ownership and final approval authority. This person is answerable for the document's quality and timeliness. There can only be one "A" per task.
- Consulted: Subject matter experts who provide input, for instance, a biostatistician reviewing a statistical analysis plan (SAP).
- Informed: Stakeholders who are kept updated on progress but are not directly involved in reviews or approvals.
To illustrate these roles in an operational context, their functions can be distinguished.
Differentiating the RACI Framework Components
| Role | Core Function | Clinical Trial Documentation Example |
|---|---|---|
| Responsible (R) | The "Doer" | The medical writer drafts the initial protocol text based on an approved synopsis. |
| Accountable (A) | The "Owner" | The Sponsor's Clinical Lead provides final approval on the protocol, making them ultimately accountable. |
| Consulted (C) | The "Expert" | A statistician is consulted to review and provide input on the statistical methods section of the protocol. |
| Informed (I) | The "Observer" | The project manager is kept informed of the protocol's status as it moves through drafting and approval. |
This table clarifies that each role serves a distinct and necessary function. The 'R' executes the task, the 'A' owns the outcome, the 'C' provides critical expertise, and the 'I' maintains awareness of progress.
The Regulatory and Operational Rationale
The requirement for such a structure is not merely an internal best practice but is also aligned with regulatory expectations. Guidelines from ICH, the FDA, and the EMA emphasize that the sponsor holds ultimate responsibility for trial conduct and data integrity. This responsibility extends directly to the quality of trial documentation, and undefined ownership is a direct risk to this principle.
Regulatory inspection reports have cited unclear roles as a root cause for significant findings. An analysis of GCP inspections found that a substantial percentage of major findings were related to incomplete or inconsistent core trial documents—issues often stemming from poorly defined drafting and quality control responsibilities. In response, many global sponsors have adopted RACI-style models for key documents to align with guidelines such as ICH E3 and ICH E6. For example, one organization reported a 25% reduction in protocol amendment cycle times after implementing a clear RACI matrix. Further information on this topic can be explored through resources on governance models and documentation training.
By assigning a single "Accountable" owner for each document, the RACI model establishes a clear line of authority that aligns directly with the sponsor's non-delegable oversight responsibilities under ICH E6(R3).
Ultimately, defining roles and responsibilities provides the foundation for quality and control in clinical trial documentation. It transitions document creation from an unstructured process into a predictable, compliant, and auditable workflow.
Mapping RACI Roles to the Clinical Trial Team
Applying the RACI framework involves translating it from a concept into a practical operational tool for the clinical trial team. This requires assigning the roles—Responsible, Accountable, Consulted, and Informed—to specific functions and individuals involved in clinical trial documentation. This process builds the operational logic that ensures clarity of purpose for all team members.
The primary objective is to create an unambiguous system of ownership. It is not uncommon for a critical document to stall because multiple individuals believe they have final approval authority, or for key expert input to be missed because a stakeholder was never formally included in the review process. By mapping roles correctly, assignments can reflect both an individual's expertise and their regulatory obligations.
Core Stakeholders and Their Typical RACI Designations
In clinical trial documentation, certain roles naturally align with specific RACI categories. While the exact configuration may vary depending on the operational model—such as the degree of outsourcing to a CRO—a logical pattern typically emerges.
-
Sponsor (Medical or Clinical Lead): This role is almost always designated as Accountable (A) for core scientific and medical documents, including the protocol and Investigator's Brochure (IB). This aligns with ICH E6 guidelines, which place ultimate responsibility for the trial's integrity on the sponsor. This accountability cannot be delegated.
-
Medical Writer: The medical writer is typically assigned the Responsible (R) role. They perform the hands-on work of drafting, editing, and compiling the document, ensuring that the content adheres to templates, style guides, and the requirements of the accountable owner.
-
Contract Research Organization (CRO): A CRO may fulfill several roles. For instance, a CRO Project Manager might be Responsible (R) for managing the document review cycle, while their functional leads in areas like clinical operations or data management are Consulted (C) for their specialized input on relevant sections.
Distinguishing Key Contributions
The effectiveness of the RACI model lies in the clear differentiation between these roles. Consider the relationship between the Sponsor's Medical Lead and a medical writer. The Medical Lead is Accountable for the scientific accuracy of the protocol, while the medical writer is Responsible for drafting it clearly and in compliance with standards. This division of labor is critical: final authority rests with the subject matter expert, while the task of content creation is handled by a skilled writer.
The same logic applies to other functions. A biostatistician is Consulted (C) on the Statistical Analysis Plan (SAP) to ensure the methodology is sound. They provide essential expert input, but the sponsor’s Head of Biostatistics is often Accountable (A) for final approval. This structure facilitates collaboration while maintaining a single, clear point of authority. For further context on how these structures are organized, the principles behind the DIA Trial Master File (TMF) Reference Model provide valuable insight.
A RACI matrix is only as effective as the operational reality it represents. The "Accountable" role must be assigned to an individual with the actual authority to approve a document and to be answerable for it during a regulatory inspection.
In large sponsor and CRO organizations, these relationships can become complex. A comprehensive RACI matrix for trial documentation might encompass 25–30 high-level tasks and involve 8–12 different functional roles, from pharmacovigilance to quality assurance. Industry analyses have shown that organizations struggling with role clarity can benefit significantly from frameworks like RACI, which can lead to faster, higher-quality decisions in a regulated environment.
Applying the RACI Model to Essential Clinical Documents
The practical application of the RACI framework involves mapping it to the creation and management of key clinical trial documents. This moves beyond theory to create an actionable roadmap that clarifies expectations for all stakeholders.
By mapping the roles and responsibilities in clinical trial documentation using a RACI model, organizations can avoid the confusion that often impedes progress. Clarifying who owns a draft, who must review it, and who has final approval authority is not just an operational enhancement; it directly supports the quality-by-design principles outlined in major guidelines like ICH E6, E3, and E9.
This structured approach is reflected in the typical hierarchical relationships within a clinical trial. The Sponsor retains ultimate responsibility, often delegating execution to a CRO, which in turn collaborates with Investigators at clinical sites.
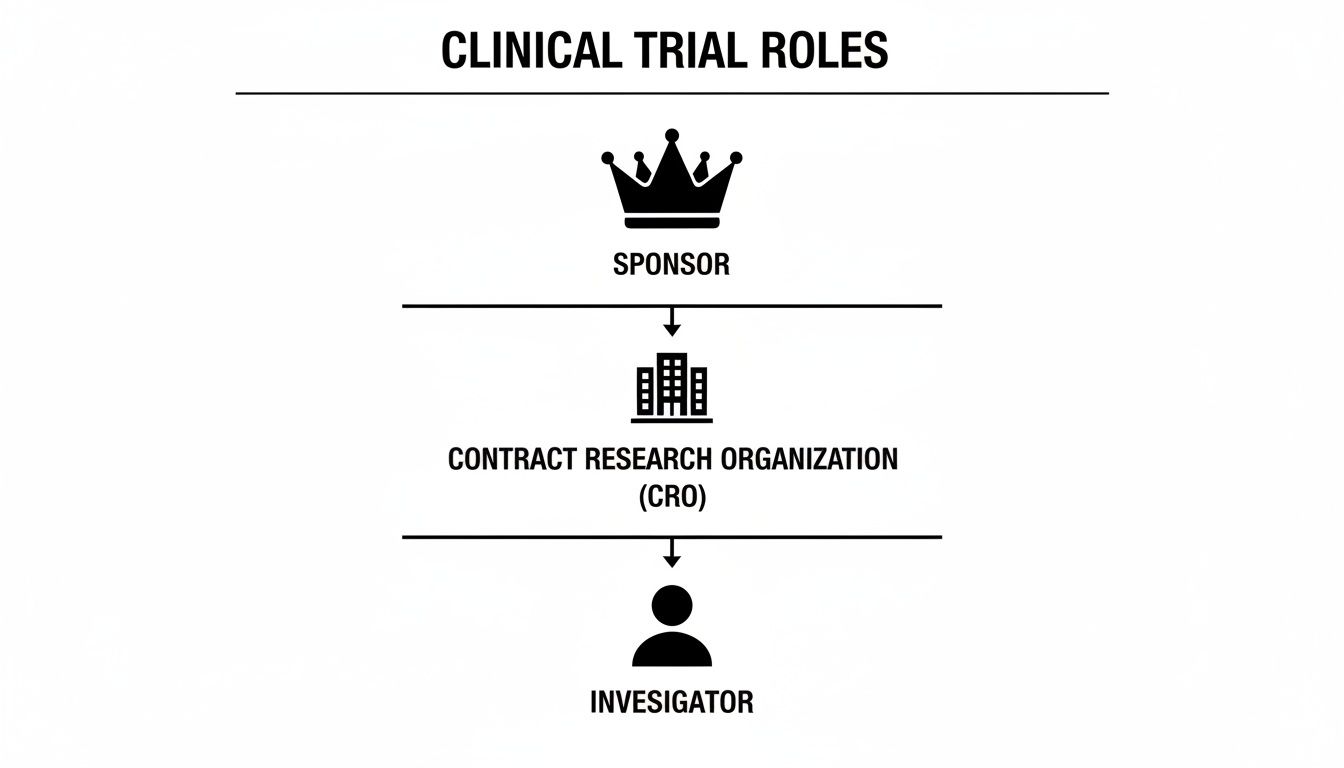
While a CRO may perform the operational tasks and an Investigator focuses on patient care, the Sponsor remains accountable for the trial's integrity and the resulting data. A RACI chart makes this explicit for every deliverable.
A Sample RACI Matrix for a Clinical Protocol
The clinical trial protocol is the foundational document for any study, integrating scientific, medical, statistical, and operational details. With input required from numerous experts, it is a document where clear role definition is critical to avoid process inefficiencies.
The following is a sample RACI matrix for protocol development.
Sample RACI Matrix for Clinical Protocol Development
This table illustrates a practical application of the RACI model for the key stages of creating and maintaining a clinical trial protocol, assigning clear roles to typical stakeholders.
| Protocol Task | Sponsor Medical Lead | Medical Writer | Biostatistician | Regulatory Affairs | QA | Clinical Operations |
|---|---|---|---|---|---|---|
| Drafting Initial Protocol | C | R | C | I | I | C |
| Statistical Section Review | I | R | A | I | I | I |
| Feasibility & Ops Review | I | R | I | C | I | A |
| Regulatory Compliance QC | I | R | I | A | C | I |
| Final Medical Approval | A | R | C | C | C | C |
| Protocol Amendment Draft | C | R | C | C | I | C |
| Final Amendment Approval | A | R | C | C | C | I |
In this example, the Sponsor’s Medical Lead is Accountable (A) for the final medical and scientific approval, holding ultimate authority. The medical writer is Responsible (R) for drafting the document, consolidating feedback, and managing the workflow.
Key Takeaway: Note that there is only one 'A' for each major approval step. The Biostatistician is Accountable for their section's integrity, but the Medical Lead is Accountable for the document as a whole. This clarity is crucial for preventing bottlenecks and satisfying regulatory expectations for clear lines of expert oversight.
Applying RACI to Other Core Documents
This same logic can be extended to other core documents in a clinical trial. While the specific stakeholders may differ, the principles remain consistent: assign a single Accountable individual, clarify who is Responsible for execution, and define who should be Consulted or kept Informed.
Below are examples for four other foundational documents:
-
Investigator's Brochure (IB): The Sponsor’s Safety or Pharmacovigilance lead is typically Accountable for the IB, particularly for annual updates incorporating new safety data. A medical writer is often Responsible for drafting, while non-clinical, clinical, and regulatory experts are Consulted.
-
Informed Consent Form (ICF): The Sponsor’s Legal department or a designated Clinical Lead is generally Accountable for the master ICF template. Clinical Operations often takes the Responsible role in adapting the master template for specific countries and sites, with legal teams and ethics committees being Consulted.
-
Statistical Analysis Plan (SAP): The lead Biostatistician is Accountable for the SAP. In line with ICH E9, they must ensure the plan aligns with the protocol’s objectives. A statistical programmer may be Responsible for drafting or performing QC on the plan.
-
Clinical Study Report (CSR): Following ICH E3 guidance, the Sponsor's Medical Lead is Accountable for the CSR’s final narrative and conclusions. The medical writer is again Responsible for the extensive task of drafting the report and integrating contributions from biostatistics, data management, and clinical operations, all of whom are Consulted.
By implementing these document-specific RACI matrices, organizations can create a transparent and auditable workflow. This is not just about operational efficiency; it is about building a robust, compliant framework where every document has clear ownership from its initial draft to final approval.
Implementing a RACI Framework in Your Organization
Implementing a RACI model requires more than creating a chart; it demands a systematic plan to integrate the framework into the organization's culture and daily operations. The objective is to transform a static matrix into a dynamic operational tool.
This initiative should begin with securing leadership endorsement. Senior management sponsorship is critical for communicating the value of the framework in enhancing compliance, improving team efficiency, and mitigating risk. Without top-level support, efforts to formalize roles and responsibilities in clinical trial documentation (raci model) may not be sustained.
Establish a Cross-Functional Governance Body
With leadership support, the next step is to form a cross-functional governance committee. This group should include representation from clinical operations, regulatory affairs, medical writing, QA, and biostatistics. Its primary responsibility is to create and standardize the RACI templates that will be used across the organization.
This committee ensures that role definitions are consistent and reflect actual operational workflows and handoffs. Involving stakeholders from the outset fosters buy-in and results in a framework that is practical and more likely to be adopted.
Develop Standardized Templates and Pilot the Program
The governance committee should focus on developing standardized RACI templates for high-impact documents such as the protocol, Investigator's Brochure (IB), Statistical Analysis Plan (SAP), and Clinical Study Report (CSR). These templates provide study teams with a consistent starting point, reducing the need to redefine processes for each new trial.
A full-scale, simultaneous rollout is often impractical. A more effective approach is to begin with a pilot program. Select one or two upcoming studies to test the RACI framework in a controlled setting. This allows for the collection of real-world feedback, identification of unforeseen challenges, and refinement of the process before a broader implementation.
A phased implementation allows an organization to learn and adapt. The goal is to demonstrate early successes from the pilot program, thereby building credibility and support for a wider rollout.
Integrate RACI into Your Quality Management System
For a RACI model to be sustainable, it must be integrated into the Quality Management System (QMS). This involves formally incorporating the RACI matrices into Standard Operating Procedures (SOPs) for document creation, review, and approval.
Data from organizations that have taken this step demonstrate its effectiveness. For example, one academic medical center integrated a RACI matrix covering over 40 trial activities into its core processes and observed a 35% reduction in protocol version-tracking errors and a 20% decrease in late safety report submissions. Research teams using RACI have reported improved task clarity in a majority of cases. These results align with findings from global CROs, where a clear RACI has been shown to reduce review cycle times and minimize unplanned revisions. More details on such outcomes can be found in publications about RACI matrix implementation findings.
By embedding the RACI framework into the QMS, it becomes part of the daily operational routine, referenced in training and verified during quality audits. For more information on setting up these processes, our guide on document review and approval workflows in clinical trials offers further insights. This integration transforms a RACI chart from a reference document into an auditable component of your compliance framework.
Avoiding Common Pitfalls in RACI Implementation
Implementing a RACI model can significantly improve clarity in clinical trial documentation workflows. However, its success depends on proper application. Merely completing a chart is insufficient; it is crucial to avoid common pitfalls that can undermine the framework's effectiveness.
A well-constructed RACI matrix should serve as the single source of truth for accountability. The following are common missteps that can hinder implementation and strategies to avoid them.
Over-Assigning Roles
A frequent mistake is assigning too many individuals to the Responsible (R) role for a single task. While collaboration is important, diluting responsibility can lead to diffusion of ownership, where no single person takes the lead.
An even more critical error is designating a committee or an entire department as Accountable (A). The "A" must be a single individual with final decision-making authority. This is essential for preventing bottlenecks and ensuring clear ownership.
The cardinal rule of RACI is one "Accountable" person per task. This singular ownership is what gives the model its efficacy, enabling progress and decisiveness.
Treating the Matrix as a Static Document
Another common pitfall is creating a RACI matrix at the beginning of a study and failing to update it. Clinical trials are dynamic; team members change, protocols are amended, and priorities shift. An outdated matrix can quickly become irrelevant.
The RACI matrix must be treated as a living document. It should be reviewed and revised at key study milestones to ensure it remains accurate.
- Following the approval of a major protocol amendment.
- When key personnel change at the sponsor or CRO.
- Before initiating a major new deliverable, such as the Clinical Study Report (CSR).
Integrating these review points into your process ensures the matrix consistently reflects the study's operational reality. For more information on managing such updates formally, refer to our guide on change control in clinical trial documentation.
Ineffective Communication and Feedback Management
Many teams encounter challenges due to ambiguity in how Consulted (C) and Informed (I) stakeholders are engaged. Without clear expectations and deadlines for feedback, 'Consulted' experts can become bottlenecks, delaying review cycles.
Conversely, over-communicating with everyone in the 'Informed' category on minor updates can create unnecessary noise and administrative burden. The solution is a well-defined communication plan that specifies how and when each group will be engaged. This ensures valuable input is gathered efficiently without overwhelming the team.
How Modern Platforms Operationalize a RACI Matrix
While a RACI matrix created in a spreadsheet is a valuable starting point, its full potential is realized when embedded into daily operational tools. Modern documentation platforms are designed to integrate the RACI framework directly into workflows, transforming it from a static chart into a dynamic, automated system.
This is where the principles behind roles and responsibilities in clinical trial documentation (raci model) become an operational reality. Instead of manually tracking signatures or sending review reminders, the platform itself can enforce the defined roles and processes.
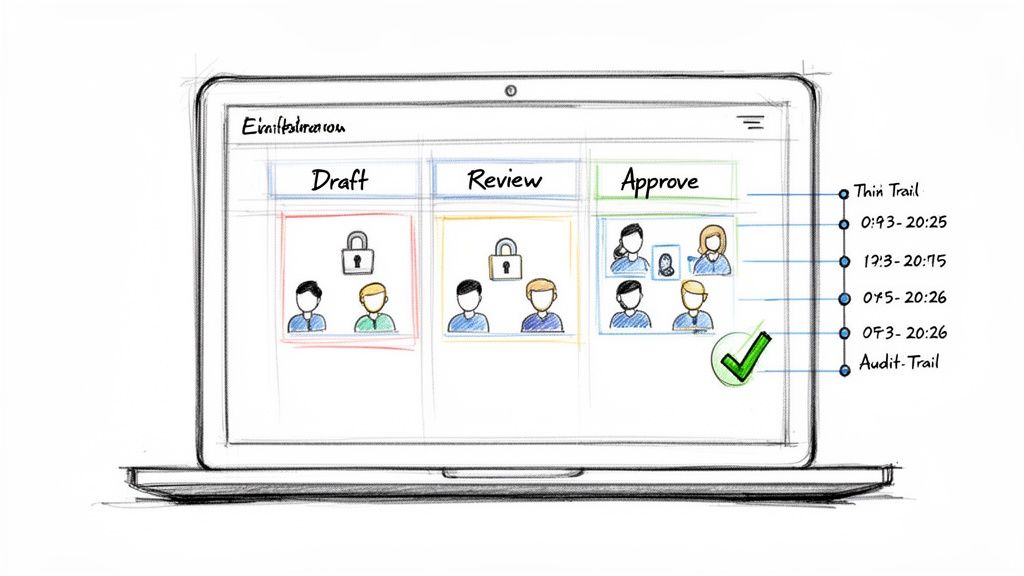
From Static Chart to Dynamic Workflow Control
Such platforms translate the logic of a RACI framework into the document lifecycle management process. This goes beyond simply hosting a spreadsheet; it involves building intelligence into the system.
These platforms achieve this through several key features:
- Role-Based Access and Permissions: The system is configured to understand who is assigned to each role. An individual designated as Responsible (R) for the CSR is granted editing rights, while someone designated as Informed (I) receives view-only access. This prevents unauthorized or accidental modifications.
- Automated Review Cycles: When a draft is ready for review, the platform automatically routes it to all individuals designated as Consulted (C). This ensures timely input from the right experts and helps prevent delays.
- Single-Point Accountability: The system can be configured to permit only the single individual designated as Accountable (A) to provide final sign-off. This hardwires the "one A" rule into the process, eliminating ambiguity about who holds ultimate approval authority.
Enforcing Compliance Through Audit Trails
A significant advantage of these platforms is their ability to support audit readiness. Every action—from initial drafting and commenting to review and final approval—is automatically captured. The system logs who performed each action and when, creating a permanent, immutable record.
For a regulator, this audit trail provides objective evidence that a controlled, predefined process was followed. It demonstrates that the sponsor maintained oversight and adhered to the quality-by-design principles central to guidelines like ICH E6(R3).
Ultimately, this integration transforms the RACI matrix from a guideline into an enforceable standard of practice. The system doesn't just recommend the correct workflow; it ensures it is followed, thereby strengthening inspection readiness and fostering a culture of accountability.
Frequently Asked Questions About RACI in Clinical Trials
The introduction of a framework like RACI often raises practical questions. The following are answers to some of the most common inquiries from clinical operations, regulatory, and medical writing teams implementing the model.
What is the distinction between "Accountable" and "Responsible" on a protocol RACI?
Understanding this distinction is fundamental to the successful application of RACI to a clinical protocol. The difference lies between doing the work and owning the outcome.
The Responsible (R) role is assigned to the "doers." This is the individual actively performing the task—for example, the medical writer who is drafting the protocol, incorporating team feedback, and managing version control.
The Accountable (A) role is for the "owner." This is the single individual with ultimate authority and who is answerable for the final result. For a protocol, this is typically the Sponsor’s Medical Lead or a comparable senior clinical expert. While multiple individuals may be responsible for various components, there can only be one person who is accountable for the overall document.
A core tenet of RACI is that each task has only one 'A'. This single point of accountability provides clarity and aligns with regulatory expectations for sponsor oversight.
How frequently should a study's RACI matrix be reviewed?
A RACI chart should not be a static document. To remain effective, it must be a living guide that accurately reflects the study's operational status.
It is recommended to review the matrix at several key points:
- During study startup: To establish clear expectations and alignment from the outset.
- Before a major protocol or SAP amendment: When the study's scope or strategy changes, the RACI may need to be updated accordingly.
- When key personnel change: The departure or arrival of a key team member at the sponsor or CRO warrants a review of role assignments.
- Prior to initiating the Clinical Study Report (CSR): This is a complex, cross-functional effort that requires crystal-clear role definitions before work begins.
Treating the RACI matrix as a dynamic document ensures it remains a valuable tool for maintaining clarity and efficiency throughout the trial lifecycle.
Can a RACI model contribute to TMF quality and inspection readiness?
Yes. A well-implemented RACI model is a valuable tool for maintaining a high-quality Trial Master File (TMF) and ensuring inspection readiness. It directly addresses a common audit finding: unclear ownership of documents.
By clearly defining who is Accountable and Responsible for each stage of a document's lifecycle—from creation and QC to final approval and filing—organizations establish a clear chain of custody. This structure helps prevent documents from being misplaced, filed incorrectly, or approved by an unauthorized individual.
When this RACI framework is integrated into a modern documentation platform, it provides a complete, auditable trail that demonstrates to inspectors that robust, controlled processes are in place.