A statistical analysis plan template is a standardized, regulatory-aligned framework that details the specific methods for analyzing clinical trial data. It serves as a comprehensive blueprint for the analysis, ensuring all procedures are prospectively defined, objective, and reproducible. This technical document expands upon the high-level statistical concepts from the clinical trial protocol, providing a granular, step-by-step operational plan.
Understanding the Statistical Analysis Plan
The Statistical Analysis Plan (SAP) is a foundational technical document in clinical development. It provides a comprehensive, in-depth description of the statistical methods to be applied to the trial data. The SAP operationalizes the statistical section of the clinical trial protocol template by providing the detailed specifications required by biostatisticians and statistical programmers to execute the analysis.
While the protocol outlines the study's overall design and objectives, the SAP specifies the precise methodology for the analysis. This separation of documents is a key element of maintaining scientific integrity in clinical research.
The Role of the SAP in Clinical Trials
The primary function of the SAP is to prospectively define the analytical approach before data unblinding. This practice aligns with the core principles detailed in regulatory guidelines such as ICH E9 Statistical Principles for Clinical Trials. By prespecifying the analysis plan, the SAP mitigates the risk of introducing bias from data-driven decisions made after observing the results.
Key functions of the SAP include:
- Providing Detailed Methodology: It specifies the exact statistical models, tests, and algorithms for all primary, secondary, and exploratory endpoints, leaving no room for ambiguity.
- Defining Analysis Populations: The document clearly outlines the criteria for each analysis set, such as the Intention-to-Treat (ITT) and Per-Protocol (PP) populations.
- Establishing Data Handling Rules: It articulates the procedures for managing missing data, handling outliers, and addressing protocol deviations.
The core principle of the SAP is to ensure that the statistical analysis is conducted in a prespecified, objective, and reproducible manner. A well-written SAP leaves minimal room for interpretation during the analysis phase, which is crucial for the credibility of the trial's findings during regulatory review.
Ultimately, the SAP serves as the operational manual for the statistical team, ensuring that every aspect of the data analysis is transparent, methodologically sound, and documented with sufficient clarity for regulatory review and potential replication. A robust statistical analysis plan template is the foundation for creating such a critical document.
Key Components of a Compliant SAP Template
An effective Statistical Analysis Plan (SAP) is a meticulously structured document where each section serves a distinct purpose. A SAP template provides the architectural blueprint for the entire analysis, ensuring all details are defined before data unblinding.
This structure acts as a guide for all stakeholders—from biostatisticians and programmers to regulatory reviewers who require a complete and defensible plan. The document flows logically from administrative details to the specific statistical models.
Foundational and Administrative Sections
The initial part of an SAP establishes context and document control. These administrative sections are essential for document management and provide the traceability required by regulatory authorities. They explicitly link the SAP to the clinical trial protocol and establish its official status.
Key components in this section include:
- Title Page: This includes the full protocol title, study identifier (e.g., NCT number), sponsor details, document version, and effective date.
- Signature Page: This provides evidence of formal review and approval by key personnel, such as the lead statistician, clinical lead, and regulatory affairs representative, creating a clear audit trail.
- Version History: As an SAP may be revised before unblinding, this section logs all changes, detailing the date, a description of the update, and the rationale for the modification.
Adherence to these foundational elements is necessary to meet Good Clinical Practice (GCP) standards, which emphasize rigorous documentation and transparency.
Core Analytical Framework
Following the administrative sections, the SAP outlines the core framework for the analysis. This part connects the high-level objectives from the protocol to the practical steps for data analysis, defining the "what" and "who" before detailing the "how."
A well-structured SAP template minimizes ambiguity by requiring the prespecification of all critical decisions—not just what endpoints are being analyzed, but precisely who is in each analysis population and how the data will be handled. Nothing should be left to interpretation.
This analytical framework is built on several pillars:
- Study Objectives and Endpoints: This section directly mirrors the protocol, restating the primary, secondary, and exploratory objectives and their corresponding endpoints.
- Analysis Datasets: It describes the specific analysis datasets (e.g., ADaM specifications) that will be derived from the raw clinical data.
- Analysis Populations: This section provides clear definitions for each group to be analyzed, specifying the exact criteria for including a subject in the Intention-to-Treat (ITT), Per-Protocol (PP), or Safety populations.
This clear foundation ensures that the statistical methods detailed later are applied correctly and consistently.
Essential Sections of a Statistical Analysis Plan
| SAP Section | Purpose and Scope | Relationship to Protocol |
|---|---|---|
| Introduction & Scope | Provides a high-level overview of the trial and states that the SAP details the methods outlined in the protocol. | Directly references the clinical trial protocol by title, number, and version. |
| Study Objectives & Endpoints | Restates the primary, secondary, and exploratory objectives and endpoints to be analyzed. | Must be an exact match to the objectives and endpoints defined in the protocol. |
| Analysis Populations | Defines the precise criteria for subject inclusion in each analysis set (e.g., ITT, PP, Safety). | Expands upon the population definitions initially described in the protocol. |
| Statistical Methods | Details the specific statistical models, hypothesis tests, and estimation methods for each endpoint. | Provides the granular "how-to" for the analytical strategies mentioned in the protocol. |
| Data Handling & Missing Data | Describes conventions for data handling, derivations, and the pre-specified plan for managing missing data. | Elaborates on the data management and statistical principles stated in the protocol. |
| Interim Analysis Plan | If applicable, outlines the timing, methods, and alpha-spending functions for any planned interim analyses. | Must align with the interim analysis strategy and stopping rules defined in the protocol. |
| Tables, Listings, & Figures | Provides mock-ups or "shells" of the planned outputs to visualize the final reporting structure. | Translates the reporting requirements from the protocol into a concrete visual plan. |
This table illustrates how the SAP builds upon the protocol, providing the detailed instructions needed to execute the statistical analysis in a prespecified and unbiased manner.
Structuring Your Statistical Analysis Plan
A well-structured Statistical Analysis Plan (SAP) serves as a clear roadmap for the entire analytical process, leaving no room for interpretation. The structure follows a logical flow, beginning with administrative details and progressing to the specific statistical methods to be applied to the trial data.
This methodical organization is essential for clarity, reproducibility, and meeting regulatory expectations. It also facilitates review by internal teams, external partners, and regulatory authorities by allowing them to quickly locate information, verify it against the protocol, and confirm that all analytical details were prospectively defined.
Title Page and Document Control
The initial pages of the SAP establish its identity and integrate it into the trial's controlled documentation system. These details are fundamental for Good Clinical Practice (GCP) and creating a verifiable audit trail.
- Title Page: This page must include the full protocol title, the unique trial identifier (e.g., protocol number, EudraCT number, or NCT number), and the name of the investigational product. It should also be clearly labeled "Statistical Analysis Plan," with the final version number and effective date.
- Signature and Approval Page: This provides evidence of approval from key stakeholders, typically including the lead biostatistician, the clinical lead or medical monitor, and representatives from clinical operations or regulatory affairs. Their signatures confirm agreement on the analytical strategy before its execution.
- Version History Log: An SAP often undergoes revisions before the database is locked. A version history table is required to track every change. Each entry must list the version number, date, a summary of the modification, and its rationale, ensuring process transparency.
Introduction and Scope
Following the administrative pages, the Introduction connects the SAP to the clinical trial protocol and defines the scope of the analysis.
This section must explicitly reference the full title, number, and version of the clinical trial protocol upon which the SAP is based. This link is critical—it demonstrates that the SAP is designed to expand on the protocol's statistical section, not alter it.
The scope must also be clarified. For instance, the SAP should state whether it covers the final analysis, an interim analysis, or an analysis for a data monitoring committee. If multiple SAPs exist for a single study (e.g., one for clinical endpoints and another for pharmacokinetics), this must be specified here.
Study Objectives and Endpoints
This section aligns the scientific goals with the statistical plan. It must contain a verbatim restatement of the primary, secondary, and any exploratory objectives from the final, approved protocol.
Next to each objective, the corresponding endpoint(s) used for its measurement must be specified. This one-to-one mapping removes ambiguity and creates a direct link between the study's research questions and the measurements to be analyzed.
A core function of the SAP is to prospectively specify every detail of the analysis. This includes not just the statistical models but also the precise definitions of analysis populations, clear rules for handling missing data, and any adjustments for multiplicity. This level of detail is essential for reproducibility and audit readiness, as expected by regulatory agencies.
The SAP is a key document for regulatory review. To be considered adequate, it must be comprehensive and specified upfront. Consensus-based recommendations outline numerous core items that an SAP should contain, covering trial objectives, analysis sets, covariate definitions, and multiplicity control strategies.
Analysis Populations
A fundamental part of any SAP is the precise, operational definition of each analysis population. Vague definitions can introduce significant bias. This section must lay out the explicit criteria for including a subject in each of the standard populations:
- Full Analysis Set (FAS) or Intention-to-Treat (ITT) Population: This population typically includes all randomized subjects. The SAP should state, for example, "all subjects who were randomized, regardless of treatment received or withdrawal from the study."
- Per-Protocol (PP) Population: This is the subset of subjects who adhered to the protocol sufficiently for their data to be considered suitable for evaluating the treatment effect. The SAP must list the exact reasons for exclusion, such as major protocol deviations related to eligibility, exposure to the investigational product, or missing key endpoint data.
- Safety Population: This group is generally defined as all subjects who received at least one dose of the investigational product or the control. The SAP should clarify how "receiving a dose" is documented and verified.
For every population, the SAP must state the treatment group to which a subject will be assigned for analysis (e.g., "as randomized" for ITT, "as treated" for Safety). Prespecifying these rules is a cornerstone of conducting an unbiased statistical analysis.
Defining Analysis Populations and Data Handling Rules
A critical function of a Statistical Analysis Plan is to prospectively define which subjects will be included in the analysis and how the data will be handled. This process involves establishing clear, unambiguous rules before data review to prevent bias, a core principle of ICH E9.
This section translates the clinical trial protocol into a concrete, operational blueprint for the statistical team. Vague definitions can lead to post-hoc decisions, which can compromise the integrity of the results and attract scrutiny during regulatory review.
Defining Standard Analysis Populations
The SAP must provide precise, operational criteria for assigning subjects to each analysis set. The definitions should be clear enough for a programmer to implement them without interpretation.
-
Intention-to-Treat (ITT) or Full Analysis Set (FAS): This population typically includes all randomized subjects. The SAP should state this clearly, for example: "The FAS will include all subjects randomized into the study, regardless of whether they received the investigational product." It should also specify that subjects will be analyzed according to the treatment group to which they were assigned.
-
Per-Protocol (PP) Population: This is a subset of the FAS who adhered to the study protocol. The SAP must explicitly list all criteria for exclusion from this set, such as major deviations from eligibility criteria, incorrect administration of the assigned treatment, or missing primary endpoint data.
-
Safety Population: This population is defined by exposure and generally includes all subjects who received at least one dose of the study drug. The SAP needs to specify the source data used for confirmation (e.g., drug accountability logs, eCRF entries) and state that subjects will be analyzed according to the treatment they actually received.
This diagram outlines the foundational structure of a robust SAP, showing the logical flow from high-level administrative details to specific analytical methods.
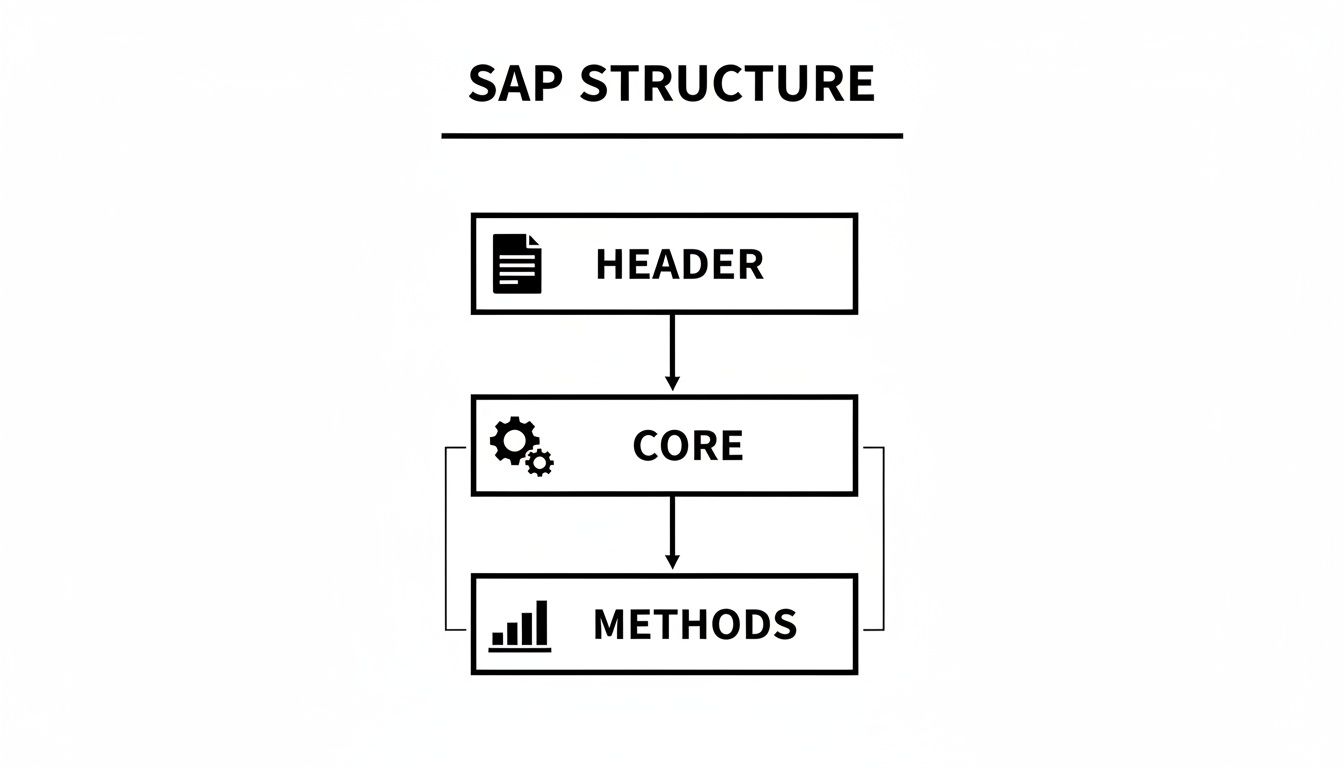
As shown, the SAP is a logically structured document where foundational rules, such as population definitions, support the detailed statistical methods that follow.
Establishing Data Handling Conventions
In addition to defining who is analyzed, the SAP must specify how their data will be managed. Documenting these rules beforehand is essential for consistency and reproducibility.
A well-drafted SAP leaves no ambiguity in data handling. It prospectively outlines the procedures for every foreseeable data issue, ensuring that the final analysis is conducted according to a predetermined, objective plan.
Key data handling rules to document in the SAP include:
-
Missing Data: Detail the strategy for handling missing values for key endpoints. Whether using Last Observation Carried Forward (LOCF), multiple imputation, or another method, the rationale for the chosen approach should be explained.
-
Outliers: Define the statistical criteria for identifying potential outliers. More importantly, specify the procedure for their review and handling during the analysis.
-
Protocol Deviations: Describe the process for identifying and classifying protocol deviations (e.g., major vs. minor) and how they will be summarized. The SAP should also link major deviations to the exclusion criteria established for the Per-Protocol population.
Specifying Efficacy and Safety Analyses
The efficacy and safety analyses section is the core of the Statistical Analysis Plan (SAP), translating the study's objectives into a step-by-step operational manual for the statistical programmer. This section must provide explicit instructions to ensure there is no ambiguity during the analysis phase.
The primary goal is to prespecify every analytical procedure to ensure the results are reproducible, transparent, and can withstand regulatory scrutiny. For efficacy, this involves defining the statistical models and tests for all primary, secondary, and exploratory endpoints. This section should be written as a technical manual, guiding the programmer so that the outputs in the final clinical study report template can be traced directly back to this plan.
Articulating Efficacy Analysis Methods
Simply naming a statistical test is insufficient. For every endpoint, the SAP must explicitly define the entire methodology, including all parameters and assumptions.
A complete specification for an efficacy endpoint should include:
- Primary Endpoint Analysis: Provide a detailed breakdown of the primary statistical model. For an ANCOVA or a mixed-model for repeated measures, specify every covariate, factor, and interaction term. The criteria for statistical significance, such as a p-value threshold of p < 0.05, must also be stated.
- Secondary Endpoint Analysis: Each secondary endpoint requires the same level of detail. If multiple endpoints are involved, describe the strategy for controlling the overall Type I error rate, such as hierarchical testing or a Bonferroni correction.
- Model Assumptions: State how the assumptions of the statistical models (e.g., normality of residuals, homogeneity of variance) will be assessed and specify the actions to be taken if those assumptions are not met.
An important standard is whether an independent statistician could perfectly replicate the analysis using only the SAP and the dataset. If the answer is no, the plan requires more detail. Vague language such as "appropriate statistical tests will be used" is a significant concern for regulatory reviewers.
Detailing Safety Data Summaries
The plan for analyzing safety data is equally critical and must be prospectively defined. Safety analyses are typically descriptive, focusing on summarizing the frequency of events and tracking changes in key health indicators.
The SAP must specify exactly how the following safety data will be managed:
- Adverse Events (AEs): Explain how AEs will be coded (e.g., using MedDRA) and then categorized and summarized by system organ class and preferred term. The plan must also provide a clear definition of a treatment-emergent AE (TEAE).
- Laboratory Data: Describe the approach for summarizing laboratory values, often using shift tables to show changes from baseline across visits. Detail how out-of-range values or clinically significant abnormalities will be identified and reported.
- Vital Signs and Other Safety Measures: Outline the methods for summarizing vital signs, ECGs, and other safety measurements. This typically involves presenting descriptive statistics stratified by treatment group and visit.
Established industry conventions often form the basis for these specifications. For example, it is common practice to use two-sided hypothesis tests with an alpha of 0.05 for primary endpoints, present categorical variables as n (%), and report continuous variables with the mean, standard deviation, and other key statistics. A well-designed statistical analysis plan template incorporates this operational consistency.
Managing SAP Version Control and Amendments
The Statistical Analysis Plan (SAP) is a controlled document that requires careful lifecycle management. Although the goal is to finalize the plan before analysis begins, amendments are sometimes necessary. Managing these changes through formal version control is a core principle of Good Clinical Practice (GCP).
This process ensures that all modifications are intentional, justified, and transparent, creating a clear audit trail for regulators. This is fundamental to the scientific validity of the trial's results, and any effective statistical analysis plan template should incorporate provisions for lifecycle management.
Finalizing the SAP Before Key Milestones
The timing of SAP finalization is as important as its content. Locking the document at the appropriate time is crucial for preventing analytical bias. Best practices and regulatory guidance align on this point: the SAP should be locked before any milestones that could influence analytical decisions.
- For Blinded Trials: The SAP must be finalized and signed off before database lock (DBL) and before the treatment blind is broken. Any amendment after unblinding will face intense regulatory scrutiny due to the potential for the analysis to be influenced by the results.
- For Open-Label Trials: Even when treatment assignments are known, it is advisable to finalize the SAP as early as possible, such as before the First Patient, First Visit (FPFV).
Finalization represents a formal agreement among the entire team on the analytical strategy before its implementation.
Documenting Amendments and Maintaining an Audit Trail
If a change to the finalized SAP is required, it must be managed through a formal amendment, not an informal update. Each change must be documented with meticulous detail to maintain a transparent and defensible audit trail.
An amendment is a formal, controlled update to a finalized document. It requires a sound scientific or operational justification, a detailed description of the change, and new approvals from the original signatories. This rigor protects the integrity of the prespecified analysis.
The amendment process includes:
- Justification: Provide a clear rationale for the change, such as a protocol amendment, new regulatory guidance, or an unexpected data issue requiring a different analytical approach.
- Version History: Update the SAP’s version history log with the version number, amendment date, a summary of the change, and the reason for the update.
- Approval: The amended SAP must undergo the same formal review and approval cycle as the original version, collecting signatures from all relevant stakeholders.
A robust regulatory document management system is instrumental in enforcing these controls, ensuring proper versioning, and preserving a complete, auditable document history. Following this systematic process is fundamental to demonstrating compliance and ensuring the credibility of trial results.
Common Questions About SAP Templates
This section addresses common questions that arise during the drafting and management of a Statistical Analysis Plan (SAP), providing a quick reference on key operational and regulatory points.
What’s the Difference Between a Protocol and an SAP?
The clinical trial protocol and the SAP serve distinct purposes. The protocol outlines the study's statistical considerations at a high level, defining the "what" and "why," such as the primary and secondary endpoints and the overall analytical strategy.
The Statistical Analysis Plan (SAP) is a more granular, technical document. It details how the analysis will be executed, specifying the statistical models, data handling rules, and precise definitions for each analysis population. A good statistical analysis plan template ensures that none of these critical operational details are overlooked.
When Should We Finalize the Statistical Analysis Plan?
The timing of finalization is critical. To maintain study integrity and prevent analytical bias, the SAP must be finalized before the data are reviewed. This is a firm regulatory expectation based on the principles outlined in ICH E9.
For any blinded study, the SAP must be finalized and signed off before the clinical database is locked and before any personnel are unblinded to treatment assignments. Locking the plan at this stage demonstrates that the analytical methods were not influenced by the study's outcomes, which is fundamental to producing objective and defensible results.
How Do We Handle Amendments to the SAP?
Any modification to a finalized SAP must be managed through a formal amendment process. This is a controlled procedure that creates a clear and transparent audit trail.
An amendment must document the rationale for the change with a clear scientific justification, note the exact date of the change, and capture the signatures of all required approvers. This level of detail is critical for regulatory transparency and is a core part of Good Clinical Practice (GCP).
Maintaining a meticulous version history within the document is essential. Every amendment must be tracked to create a complete history of the analytical plan that can withstand regulatory scrutiny.