A Guide to Medical Writing for Clinical Trials
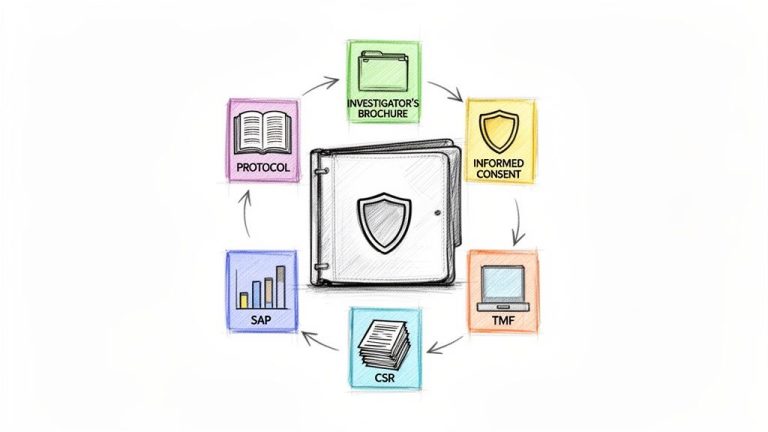
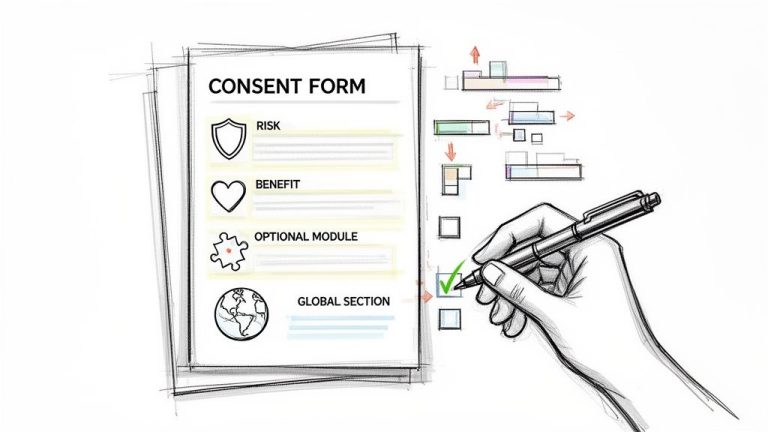
Medical writing for clinical trials is a specialized discipline focused on preparing clear, structured, and compliant documentation required by regulatory bodies such as the FDA and EMA for the approval of new therapies. Its primary function is to translate complex…